
Interactions among species are an integral aspect to the evolution of life. Some of the most widespread and consequential species interactions are the relationships between multicellular eukaryotes and microorganisms. Vertebrates serve as habitats for thousands of microbial lineages, which in turn have opened up new adaptive trajectories for their hosts. Nearly every dimension of vertebrate biology, from development to digestion to behavior, has become deeply integrated with symbiotic microbial communities. Despite the ubiquity and profound effects of the relationships between hosts and microorganisms, many fundamental questions remain unanswered. Which microorganisms coevolve with hosts? What are the genetic bases of the intimate partnerships between host and microbial lineages? What ecological processes drive the compositions of host-associated microbiotas? How have microbes swayed the course of host adaption and diversification? Our work is aimed at answering these and related questions through a combination of field studies and experimental manipulations in the lab.
Vertebrate-bacteria co-adaptation
The gut microbiota is integrated with vertebrate phenotypes ranging from gene expression to the development of the immune system to behavior, but the history of reciprocal adaptations underlying these integrations is unclear. The concerted diversification of nuclear, mitochondrial, and gut bacterial genomes provides an exciting framework for studying coadaptation between vertebrates and their bacteria. We are employing gene-editing and microbiota-transplant approaches to study the genetic bases and phenotypic consequences of co-adaptation between gut bacteria and host species. This work utilizes gnotobiotic animals, host cell lines, and gut bacterial isolates to better understand how host genomes and microbial metagenomes interact to shape phenotypes and fitness.
Phenotypic effects of microbiome variation
Microbiomes can diversify alongside host lineages, but the effects of microbiome changes on host phenotypes are poorly understood. For example, we have found that lineages of gut bacteria have been maintained exclusively within hominid lineages for hundreds of thousands of host generations, but many of these ancestral gut bacteria have been lost from human populations. Reductions in ancestral microbial diversity are particularly severe in industrialized societies, but the consequences of these microbial losses for hosts remain unknown. We are applying a variety of experimental approaches to test the effects of recent changes in the human microbiota on human cellular and tissue phenotypes.
Vertebrate-bacteria co-adaptation
The gut microbiota is integrated with vertebrate phenotypes ranging from gene expression to the development of the immune system to behavior, but the history of reciprocal adaptations underlying these integrations is unclear. The concerted diversification of nuclear, mitochondrial, and gut bacterial genomes provides an exciting framework for studying coadaptation between vertebrates and their bacteria. We are employing gene-editing and microbiota-transplant approaches to study the genetic bases and phenotypic consequences of co-adaptation between gut bacteria and host species. This work utilizes gnotobiotic animals, host cell lines, and gut bacterial isolates to better understand how host genomes and microbial metagenomes interact to shape phenotypes and fitness.
Phenotypic effects of microbiome variation
Microbiomes can diversify alongside host lineages, but the effects of microbiome changes on host phenotypes are poorly understood. For example, we have found that lineages of gut bacteria have been maintained exclusively within hominid lineages for hundreds of thousands of host generations, but many of these ancestral gut bacteria have been lost from human populations. Reductions in ancestral microbial diversity are particularly severe in industrialized societies, but the consequences of these microbial losses for hosts remain unknown. We are applying a variety of experimental approaches to test the effects of recent changes in the human microbiota on human cellular and tissue phenotypes.
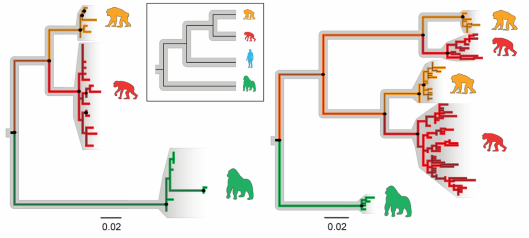
Some of the bacterial lineages that cospeciated with hominids have been lost from humans living in the United States. Inset displays the phylogenetic relationships among chimpanzees (orange), bonobos (red), humans (blue) and gorillas (green). Colored phylogenies portray the relationships within two clades of cospeciating Bacteroides present within wild-living apes but missing from humans. Scale bars indicate synonymous divergence of DNA gyrase subunit B genes, and black dots mark nodes supported by >50% of bootstrap replicates.
Microbial transmission within and between vertebrate lineages
The observation that vertebrates and their gut bacteria can co-speciate raises questions about the mode of inheritance of gut bacteria across generations. Placental mammals receive their first microbes from the mother at birth (Dominguez-Bello et al., 2010 PNAS), but throughout life they are continuously seeded by microorganisms from external sources, including other conspecifics (Tung et al., 2015 eLife; Moeller et al., 2016 Sci. Adv.). The inheritance of the gut microbiota is likely a composite of vertical and horizontal transmission, but determining which bacterial lineages are inherited vertically versus horizontally has been challenging. A recent project quantified the fidelity of bacterial genera in the gut microbiota of Mus musculus by monitoring vertical and horizontal transmission from mother to offspring under lab conditions (Moeller et al., 2018 Science). Additional investigations of microbiota inheritance are focusing on long-term monitoring of vertebrates in the wild.
The observation that vertebrates and their gut bacteria can co-speciate raises questions about the mode of inheritance of gut bacteria across generations. Placental mammals receive their first microbes from the mother at birth (Dominguez-Bello et al., 2010 PNAS), but throughout life they are continuously seeded by microorganisms from external sources, including other conspecifics (Tung et al., 2015 eLife; Moeller et al., 2016 Sci. Adv.). The inheritance of the gut microbiota is likely a composite of vertical and horizontal transmission, but determining which bacterial lineages are inherited vertically versus horizontally has been challenging. A recent project quantified the fidelity of bacterial genera in the gut microbiota of Mus musculus by monitoring vertical and horizontal transmission from mother to offspring under lab conditions (Moeller et al., 2018 Science). Additional investigations of microbiota inheritance are focusing on long-term monitoring of vertebrates in the wild.